Welcome to our blog center
You can find tips, basics, guide and more information about manufacturing processes and materials that we have learned from practice here.


Aluminum Metal Stamping: Types, Process, Applications, and Industries
This blog post shows whatever you want to know about aluminum stamping: process, types, applications and industries using aluminum stamping parts.

Plastic 3D Printing Showdown: SLS vs. SLA
3D printing has transformed the design and production of parts, greatly facilitating rapid prototyping and even custom production. Two very important types of 3D printing
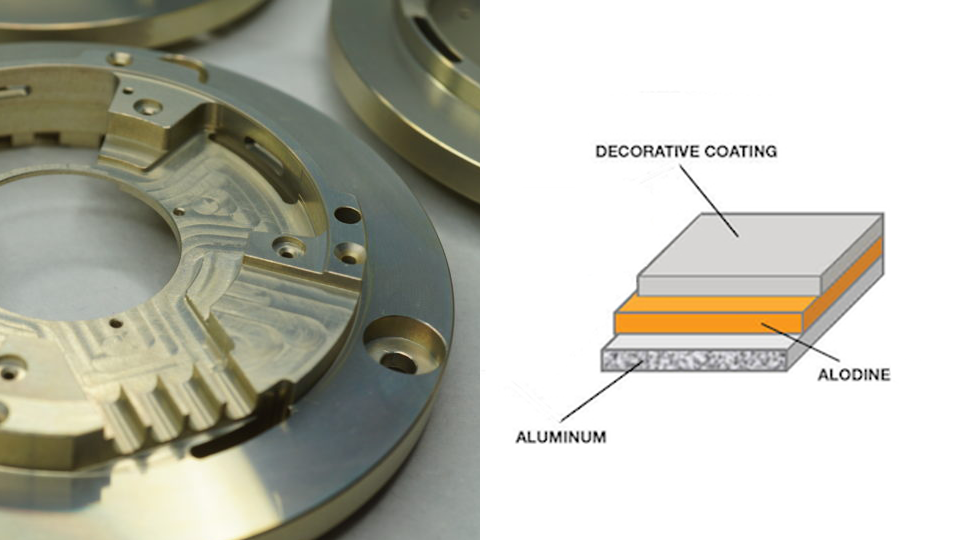
Alodine Coating 101: A Comprehensive Guide
Alodine coating, also known as chromate conversion coating, is a widely used method for enhancing the surface properties of aluminum alloys.
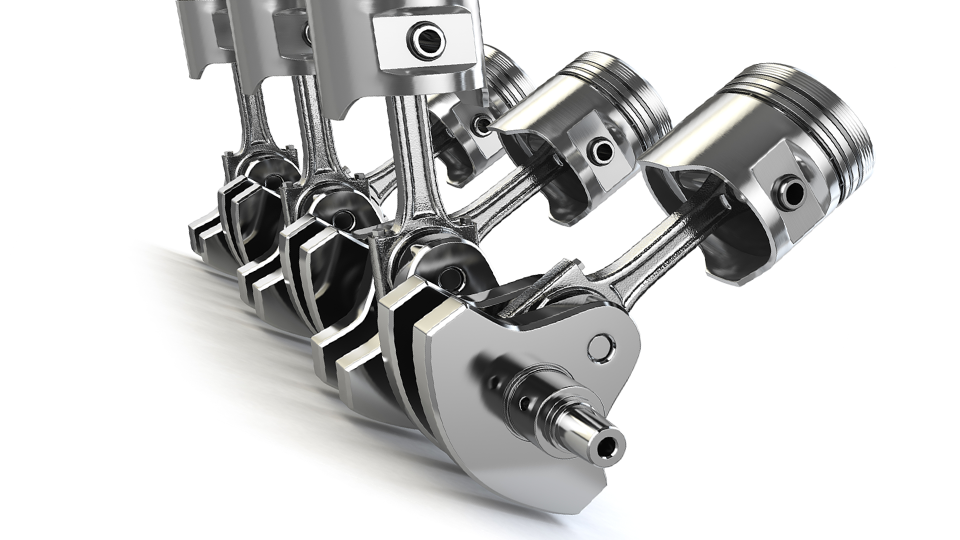
Mastering Piston Machining: Techniques, Materials and Finishes
he piston machining requires high precision and advanced techniques to be able to achieve the most demanding standards in performance. This article will approach the materials used, machining processes involved in the manufacture of pistons.

The General CNC Machining Tolerance: ISO 2768-mk
Tolerances are critical to ensure that parts meet design specifications and can be assembled properly. To help you better understand this concept, here is an
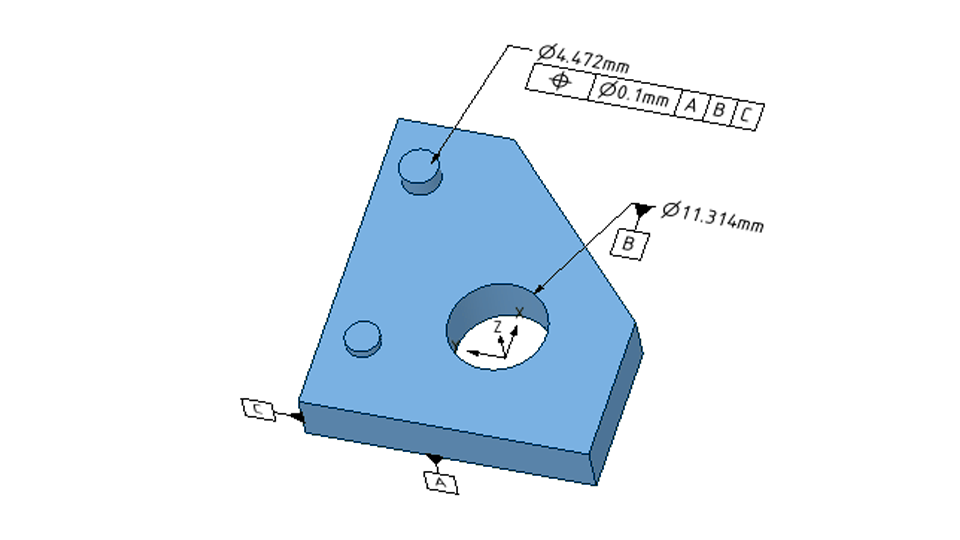
What are Engineering Tolerances and How are They Classified?
Engineering tolerances define the acceptable limits of variation of physical dimensions, shapes, or properties of a component. These tolerances ensure that parts fit and function properly in an assembly and that the final product meets the specified requirements.
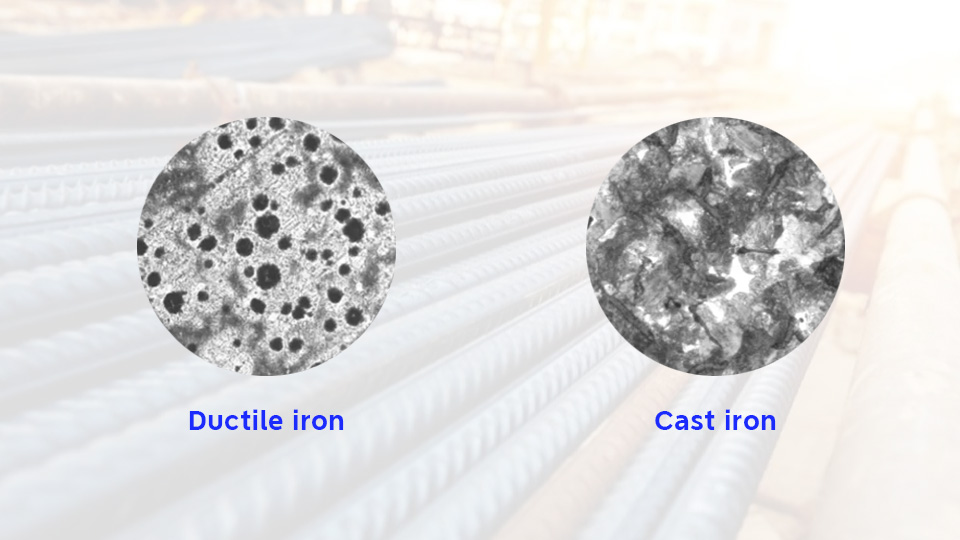
Gray or Ductile Iron? Cast Iron Types Compared for Industrial Use
The term “cast iron” is the generic name for iron-carbon alloys containing more than 2% carbon. The subclasses of cast iron are numerous, some of them are gray iron and ductile iron.

How to Chrome Plate Aluminum and its Alloys
Chrome plating refers to the process of applying a thin layer of chrome onto another metal(like aluminum). This can increase hardness and durability on the surface, prevent corrosion, and even make it easier to clean.
SIGN UP FOR US!
Enter your email address to subscribe to our newsletter!

On-demand Manufacturing Services
1 to 1000+ pcs metal or plastic parts, global delivery as fast as 7 days.
