Electroplating Services
Our electroplating services will enhance the durability and corrosion resistance of your metal parts, and meet the stringent requirements of many industries.
Electroplating Specification
Electroplating is the process of depositing a metal coating, such as nickel, chrome, or zinc, by using an electric current onto a substrate. Most commonly done on aluminum and stainless steel, it increases corrosion resistance and durability of the substrate material.
Plated Materials |
Plating Options |
Aluminum, Stainless Steel, Steel |
Nickel, Chrome, Zinc, Gold, Silver |
|---|
Types of Electroplating Processes
The processes included within electroplating include nickel, chrome, zinc, gold, and silver, each type providing different advantages in terms of resistance to corrosion, durability, and looking nice, which can be applied industrially onto various materials.
Nickel Plating
Nickel plating deposits a nickel layer onto a substrate, offering a shiny finish that protects against corrosion and wear.
Chrome Plating
Chromium plating applies a thin layer of chromium to a metal surface, providing a highly reflective, durable finish.
Zinc Plating
Zinc plating applies a thin layer of zinc to a substrate to protect against rust and corrosion and offers a matte or shiny finish.
Gold Plating
Gold plating deposits a thin layer of gold onto a substrate, offering an attractive, durable finish that resists corrosion and tarnishing.
Gold Plating
Silver plating applies a layer of silver to a metal surface, creating an attractive, smooth finish that resists corrosion and oxidation.






Advantages
- Add protective layer to prevent rusting and corrosion, extend the lifespan of metal parts.
- Gold or silver plating can enhance electrical conductivity, which is vital for electronic components and connectors.
- Provide wear resistance, ensuring products withstand regular usage and adverse conditions.
Notes
- Electroplated coatings are often not as thick as mechanically deposited coatings.
- Electroplating can introduce stresses into the workpiece.
How Electroplating Works
Electroplating is the process of applying a metal coating to a workpiece by means of electricity. The item is first properly cleaned of dirt, oils, or oxides for a smooth surface to which the metal can adhere. Following this step, the workpiece is submerged into a bath containing a solution of metal salts, where it becomes the cathode, with a metal electrode serving as the anode. The electric current passing through the solution attracts metal ions onto the surface of the plated object and deposits a solid layer of metal. Items are thoroughly rinsed, dried, and carefully inspected following plating for quality and correct finish.
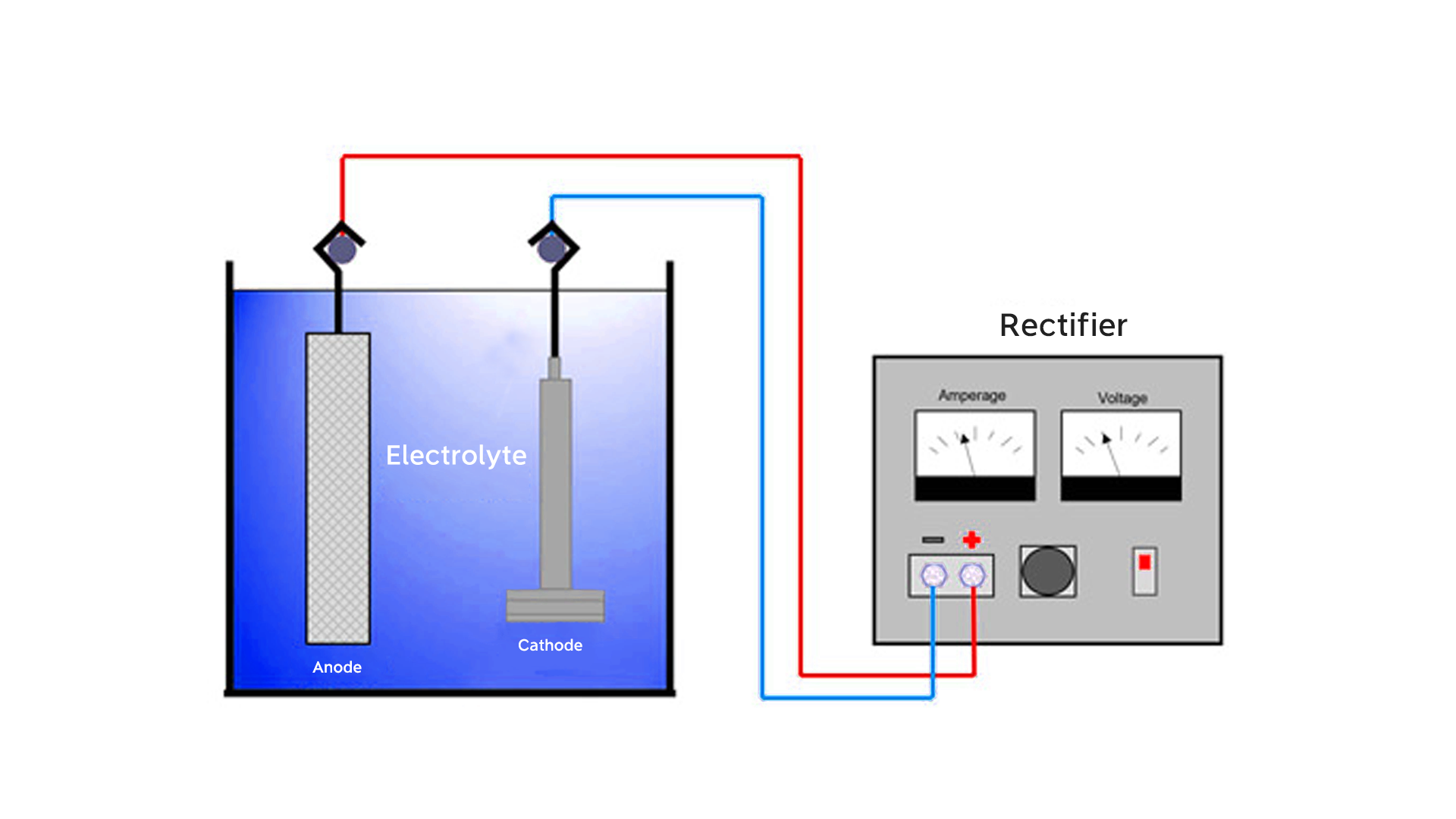
- It is necessary to evaluate the thickness of the plating for all key dimensions and tolerances.
- Electroplating requires the use of current. This implies that the component's overall geometry will affect the current circulation across its surface area, frequently in an unpredictable manner. Some sophisticated plating techniques, however, can guard against excessive plating accumulation on bends, threads, and sharp edges.
- When selecting plating materials, you should consider the required properties of the finished product, such as strength and resistance to corrosion.
Electroplating Design Considerations
What is the difference between electroplating and anodizing?
Electroplating is a process whereby a metallic coating, such as gold, silver, or chrome, is deposited onto a substrate using the action of electric current to alter its surface characteristics, including durability and appearance. On the other hand, anodizing finishing is an electrochemical process that thickens the natural oxide layer of metals like aluminum to give them more resistance to corrosion and wear and to provide an opportunity to dye them.
What is the difference between electroplating and electroless plating?
Electroplating uses an electric current to coat a surface with a thin layer of metal and is often used to improve appearance, resistance to corrosion, or conductivity. On the other hand, electroless plating relies on a chemical reaction to deposit metals on a substrate without electricity; this way, it offers a more uniform coating over complex or non-conductive surfaces.
How long does gold electroplating last?
The longevity of gold electroplating depends on several factors, such as the thickness of the plating, the frequency of use, and any exposure to harsh conditions. Gold plating can last from a few months up to a couple of years on jewelry and decorative items, while thicker coatings or more durable applications—for example, electronics—can have a longer life span with proper care and maintenance. Continual exposure to friction, moisture, and chemicals can significantly reduce this life expectancy of plating.
